Spring 2015, Prof. Mason
3 March 2015

We started today's class with an experiment. An aluminum can filled with water is heated up. Then the can is removed and put upside down into a cold water. The can then immediately implode because the water inside turned into water vapor when it was heated, then it changed back to water when it cooled. So it changed the volume and the pressure rapidly, causing the can imploded quickly.
When the can is not filled up with water and we do the same thing to the can, the can will not impose at all. Instead, it pulls the water to the top.
Pressure units that we know are Pa, Psi, mmHg, N/m(square), atm, tore, and bar. Given a density of 1 kg/m(square) at 10 km below the water, we needed to calculate how much pressure there is. Using the formula, we multiplied density, height, and gravity, and found the result to be 10,000 kg/m.s(squared).
Comparing Pressure to Volume using logger pro:
We were given a syringe and set of sensors. After setting up the logger pro and connecting the sensors, we needed to find the pressure when the syringe is pushed from 20cc to 6cc. To collect the Volume data, the process is:
- Click on the "clock" sign
- Change entry option from "time" to "events"
- Every time you push down the syringe by 2cc, click on the wheel icon, and enter the cc.

There are many types of pressure formulas, but the one that we would be using the most is using R and Kb. The relationship of them created avogadro's number, which we have known from Chemistry class.
We also find the units of pressure to be kg*m/s(square).
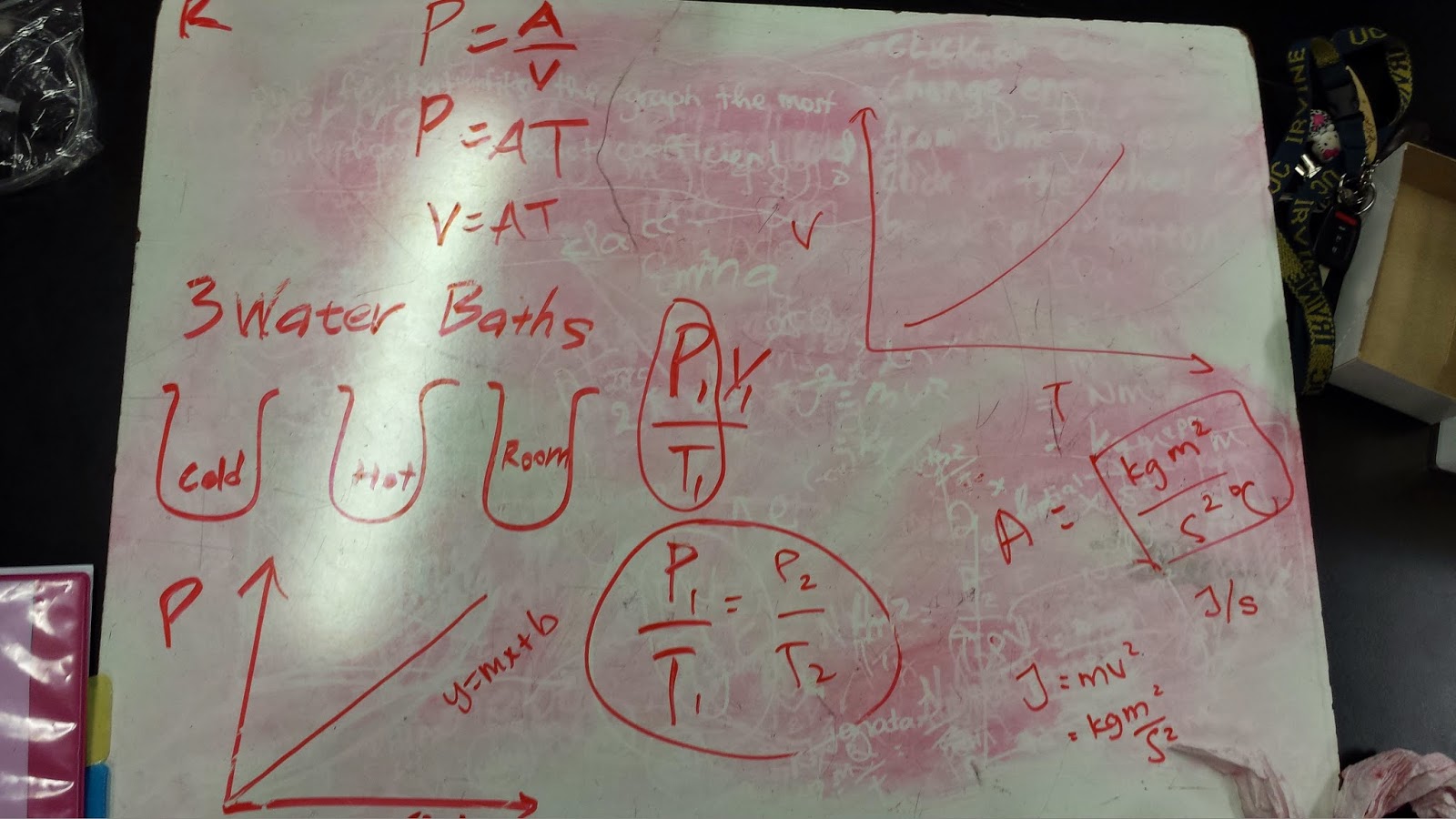 The next experiment, we obtain 3 water baths in cold, hot and room temperature. From this experiment, we wanted to see the relationship between the pressure and temperature. Our prediction came out about the same as the experiment. They have a linear proportional relationship as seen in the graph that is sort of linear.
The next experiment, we obtain 3 water baths in cold, hot and room temperature. From this experiment, we wanted to see the relationship between the pressure and temperature. Our prediction came out about the same as the experiment. They have a linear proportional relationship as seen in the graph that is sort of linear.
Then, we wanted to see the relationship
between volume and temperature from the same experiment. When we changed the variable, we found the relationship between volume and time to be about the same as pressure. Thus, we found then to be inversely proportional.

To confirm the relationship between pressure and volume, we put a balloon in an isolated container. We let some of the air out for sometimes and watch what was happening to the balloon. When we let the air out, the balloon grows in size, but when we let the air in again, the balloon shrink again. This shows the relationship between pressure and volume. The balloon tries to balance the pressure by increasing its volume.
We are given a hollow cylindrical with radius 1.5 m and height 4.25 m. At the surface, it is 25*C, with 1 atm pressure. It is going 225 m under the water where the temperature is 15*C. We needed to find how much water is being pushed out when the cylinder is going in. So we started off calculating the total pressure by adding atmospheric pressure and the water pressure. This equation gives us the height of the empty space inside the cylinder. Then we subtract the height of the cylinder by the height of that empty space.
~THE END~








No comments:
Post a Comment